Laboratory Chemical Search
Barium Acetate
Also known as Barium Salt
| H.S.Code | 29152990 | CAS No. | 543-80-6 |
| Molecular Formula | C4H6BaO4 | Molecular Weight | 255.41 |
| Physical state | Solid | Refractive index | 1.64 |
| Odour | Odorless | Solubility in water | 72 g/100mL (20 °C) |
| Density | 2.47 g/cm³ | Flash point | |
| pH value | 7.0 - 8.5 | Melting Point | 450°C |
About Barium Acetate
Barium acetate is the salt of barium and acetic acid. Barium acetate is generally produced by the reaction of acetic acid with barium carbonate. The reaction is performed in solution and the barium acetate crystallizes out.
Barium acetate is toxic to humans, but has use in chemistry and manufacturing. In chemistry, it is used in the preparation of other acetates; and as a catalyst in organic synthesis. Barium acetate is used as a mordant for printing textile fabrics, for drying paints and varnishes and in lubricating oil.
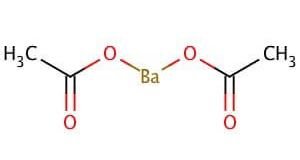
Barium Acetate Specifications
| Packaging | 500 gm, 1 kg |
| Form | Powder |
| Colour | White |
| Grade | LR / AR |
| Hazard Class | 6.1 |
| Storage | Store at +2°C to +30°C |
| Shelf Life | 60 Months |

500 gm